Recently the Drug Enforcement Administration (DEA) has been reporting seizures of fake Adderall...
Intro
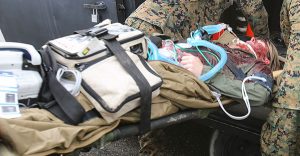 Since the American Revolution, the current landscape and capabilities of field first-aid have dramatically changed. Technology and innovation have provided new means for effectiveness and readiness of wound care for soldiers in the middle of combat. In a 2007 study conducted by military medical experts, treatment records for all Special Operations Forces within the US Army between 2001-2004 were reviewed to identify whether the soldiers’ wounds were “nonsurvivable” or “potentially survivable.” Of the 82 deaths reviewed, a majority were declared nonsurvivable; however, 50% of those with potentially survivable injuries died from truncal hemorrhage, the #1 cause of potentially survivable deaths in combat. Even 11 years later, the threats of hemorrhage and uncontrolled blood loss are still pressing issues. A 2013 study stated that of the 4,596 combat deaths in Iraq and Afghanistan between 2001 and 2011, these threats were the leading cause of death in 90% of battlefield cases. So, the question is: what solutions are there to combat these threats? How can we turn those potentially survivable deaths into soldiers that do survive?
Since the American Revolution, the current landscape and capabilities of field first-aid have dramatically changed. Technology and innovation have provided new means for effectiveness and readiness of wound care for soldiers in the middle of combat. In a 2007 study conducted by military medical experts, treatment records for all Special Operations Forces within the US Army between 2001-2004 were reviewed to identify whether the soldiers’ wounds were “nonsurvivable” or “potentially survivable.” Of the 82 deaths reviewed, a majority were declared nonsurvivable; however, 50% of those with potentially survivable injuries died from truncal hemorrhage, the #1 cause of potentially survivable deaths in combat. Even 11 years later, the threats of hemorrhage and uncontrolled blood loss are still pressing issues. A 2013 study stated that of the 4,596 combat deaths in Iraq and Afghanistan between 2001 and 2011, these threats were the leading cause of death in 90% of battlefield cases. So, the question is: what solutions are there to combat these threats? How can we turn those potentially survivable deaths into soldiers that do survive?
Federal Resources sat down with MRO industry and market expert Tim Langan from SERE Industries to weigh-in on the current field first-aid situation and what solutions are out there to modernize these capabilities.
Q&A
Q1: Let’s address the scope of the current capabilities in field first-aid. What combat conditions/issues do soldiers currently face?
A: The first concern is stopping life threatening hemorrhage, which is the number one preventable killer on the battlefield. Hemorrhagic shock is still the main cause of battlefield mortality and the second most prevalent cause of mortality in civilian trauma. Secondly, advanced SOP’s with respect to certifications for bi-annual training to all personnel on Individual First-Aid Kits (IFAK) would be beneficial as well to our military.
Q2: What operational standards are currently in place for soldiers to deal with these issues?
A: “The use of hemostatic dressings and agents is one of the main advancements achieved in recent decades. However, it can be claimed that the ideal hemostatic has not been recognized yet; therefore, this topic needs to be brought into focus and further addressed” [1]
Currently, some of the treatment options for external battlefield wounds include Chitosan, Kaolin, and Zeolite. Chitosan, derived from shrimp shells, can cause carbamate toxins and anaphylaxis as shrimp is cross-reacting allergen. Kaolin, a Chinese clay, runs the risk of skin irritation and respiratory exposure that can tear from the cell wall. Zeolite, a volcanic rock, is associated with exothermic reactions, as well as or vascular complications. “There were mild edematous and vacuolar changes in nerve samples” that were treated with Zeolite.
There are other combination-product wound dressings for external wounds that are effective in the short run, but not so much in the long run. One of these combination-products is thrombin, which can be useful for controlling minor bleeding from capillaries and small venules, but ineffective and not indicated for massive or brisk arterial bleeding.
Wound dressings for external wounds that include zeolite, various clays (bentonite or kaolin are principal examples), bandage, and sponge products with other combined products mentioned above can cause further hemorrhaging upon removal.
It’s important to note: All of these current products make life more difficult for the medical professionals who have to deal with the injured patient. Subsequently these products traumatize the fragile and damaged tissue further and have to be removed or irrigated within 24 hours.
Q3: What kind of technology/techniques are available to modernize wound management and who do they apply to?
A: The “Samaritan” compound can be applied to all first-aid or wound management needs. The product can be color coded for specific injury markers, and /or completely opaque or transparent for numerous applications or classifications in wound treatment. The Samaritan product can deliver a "bioactive" layer, of specific impregnated Nano particles or special elements to distribute antibiotics, pain medication, treat for shock and to prevent systemic fungal and/or bacterial infections with broad-spectrum activity. Samaritan does not need to be irrigated every 24 hours or removed. It can be left on the wound for up to 29 days.
Q4: How does the Samaritan work? What are the advantages of integrating it if the military [or law enforcement] chose to do so?
A: The “Samaritan” product is a lifesaving revolutionary game changer. Essentially Samaritan is a fusible flexible proprietary compound used primarily for combat casualty care to STOP BLEEDING, dress wounds, or prep the area for further medical treatment to begin promoting effective cellular healing. Samaritan is a fully biodegradable, non-breathable product that and adheres to the skin, with no separate taping needed. Samaritan has a superior super effect, that acts as “molecular glue” which reinforces the hemostatic process providing rapid and accelerated coagulation or blood clotting that quickly stops and reduces the amount of bleeding present. Samaritan can be easily applied on open wounds such as any skin injury caused by abrasion with road surfaces, explosions, or common battlefield scrapes. When applied Samaritan flows into microscopic grooves. It does not tear away from the wound or cell wall and can be easily removed in one piece. The blood vessels remain occluded after removal. Samaritan can be seen inside the wound as Barium can be added for x-raying purposes.
The benefits of Samaritan treatment are multi-dimensional and will overlap across a broad spectrum of environments or marketing sectors. The Samaritan product and individual delivery systems will solve dynamic medical and health-related problems that compromise the safety, or deter the mission performance of Soldier, Sailor, and Aviator. The product will provide multiple applications for use in proper crisis management in diverse areas of operation, especially as a means to provide rapid triage for mass casualty care for first responders and civilians around the world.
BASIC product ingredients are all FDA cleared. The general product is meeting all 5 requirements for future full FDA approval:
- Showing superior effectiveness
- Avoiding serious side effects of an available treatment
- Improving the diagnosis of a serious disease or bleeding events where early diagnosis results in an improved outcome
- Decreasing a clinically significant toxicity of an available treatments such Quick Clot
- Addressing an expected public health need and battle field trauma care
SHELF LIFE OF THE BASIC SAMARITAN
- Will be stated on our Instructions 2 +plus years without Medication.
- Contained properly, stored product will last beyond 5 years. For reference Samaritan Med 2.0 product is in its seventh year and still effective
EXTREME TEMPERATURES
- Does not Melt; Product not Temperature sensitive
- 392 F -58 degrees below zero. Or 200 C/-50 Product works better at lower temp.
- All materials in the product formula are predominantly organic in nature and begin decomposing around 350 - 700 deg C
For more information on the Samaritan treatment, please contact scott.graham@julianneg3.sg-host.com.
Q5: How do you see the field of MRO evolving? Do you think the R&D in this space is keeping up with the threat climate?
A: The goal should be to provide the first choice for high quality and innovative medical products, logistics support and training along with advanced SME liaison to support U.S. and allied forces deployed to U.S. European and U.S. Central Commands. If our customers can work with industry to enhance product application for mitigation of medical threats and collaboration wherever Soldiers are, whenever needed. Basically, the goals should be to improve and sustain Warfighter health and performance under all conditions.
SAMARITAN EAR PRO (SEP-X) Samaritan Polymer is the next generation of cutting edge battlefield hearing protection. This product will change how the warfighter protects his/ her hearing in the current battlefield environment.
- Samaritan is a gel-based polymer that can be easily administered to the ear by the user without the need of a specialist.
- Samaritan has an easy application process. It cures to a polymer-based gel within 30 seconds. The warfighter can still hear ambient sound, while still protecting the inner eardrum. It can also work with all over the ear radio communication products like Peltor Headset to include other over the ear communication systems.
- Samaritan can be used with inner-ear radio communication products, eliminating pressure to existing bone connection mics that press against the Soldier’s inner ear.
- Samaritan once applied will fit comfortably under ballistic and military helmets eliminating any and all irritation or physical distraction to the warfighter.
CREATES A CUSTOM PROTECTIVE AND ACOUSTIC NOISE BARRIER FOR THE EAR
- No less than 1 CC per Soldier ear
- Package contains 1 pair of Samaritan 'plugs', re-useable.
- Hypo-allergenic, nontoxic and water repellent.
- Noise Reduction Rating of 30 or more Decibels when used as directed.
- Cure time 30 seconds
- Protect your ears from water, cold air, dirt and bacteria without compromising your hearing or balance.
- With no hard core, these super soft silicone ear plugs mold to fit your ear canal perfectly and offer a very comfortable fit while providing a high level of water protection.
- Antimicrobial and Hypoallergenic - Inhibits allergic reactions and growth of harmful bacteria
- Allows for asymmetrical ear dimensions, and the configuration is able to adapt and fit snugly
- Form fit reduces headaches
Sources
[1] Overview of Agents Used for Emergency Hemostasis
Hadi Khoshmohabat,1 Shahram Paydar,2,3 Hossein Mohammad Kazemi,1 and Behnam Dalfardi1,2,*
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4869418/
Holcomb, J. B., McMullin, N. R., Pearse, L., Caruso, J., Wade, C. E., Oetjen-Gerdes, L., … Butler, F. K. (2007). Causes of Death in U.S. Special Operations Forces in the Global War on Terrorism: 2001–2004. Annals of Surgery, 245(6), 986–991. http://doi.org/10.1097/01.sla.0000259433.03754.98
Pryor Medical Devices Wins $14.3MM Contract for REBOA Research. (n.d.) >The Free Library. (2014). Retrieved Apr 11 2018 from https://www.thefreelibrary.com/Pryor+Medical+Devices+Wins+%2414.3MM+Contract+for+REBOA+Research.-a0434324194
Military Times. (2017, August 08). Study: 25% of war deaths medically preventable. Retrieved April 11, 2018, fromhttps://www.militarytimes.com/2013/03/29/study-25-of-war-deaths-medically-preventable/
https://en.wikipedia.org/wiki/Thrombin#cite_note-pmid17660072-23
Chapman WC, Singla N, Genyk Y, McNeil JW, Renkens KL, Reynolds TC, Murphy A, Weaver FA (August 2007). "A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis". Journal of the American College of Surgeons. 205 (2): 256–65. doi:10.1016/j.jamcollsurg.2007.03.020. PMID 17660072.
https://www.researchgate.net/post/Is_allergy_a_real_issue_in_chitosan_from_shellfish_origin
https://www.researchgate.net/profile/Staffan_Palm2
